Varicella-Zoster Virus DNA, Qualitative, Real-Time PCR QVZVQL
| Method(s) | Real-Time Polymerase Chain Reaction |
|---|---|
| Specimen Required | |
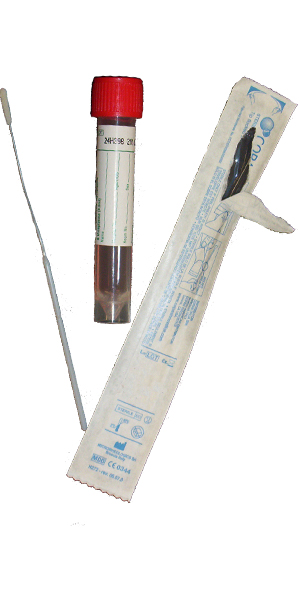
| Collect | One 3 mL lavender (EDTA) or CSF or bronchial wash/brush in sterile, plastic, leak-proof container or Lesions swab in green (VCM) top tube or equivalent UTM or M4 or 0.5 Ml eye fluid (vitreous) in sterile, plastic, leak-proof container |
| Transport | 1 mL whole blood refrigerated (Min: 0.3 mL eye fluid, 0.3 mL all other specimens). |
| Stability | Whole blood Ambient: 48 hours Refrigerated: 7 days Frozen: Unacceptable All other specimen types Ambient: 48 hours Refrigerated: 7 days Frozen: 30 days |
| Unacceptable Conditions | Heparin whole blood |
| Schedule | Daily; Report available: 4-5 days |
| Billing Code | 9011755 |
| CPTCode | 87798 |
| Notes | Performing Laboratory Quest Diagnostics Nichols Institute-Chantilly VA |
| Preferred Specimen Collection Device(s) | |