Chlamydia trachomatis Culture CHLTC
| Method(s) | Cell Culture/Immunofluorescence |
|---|---|
| Specimen Required | |
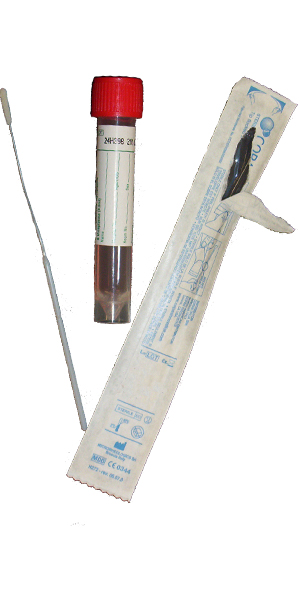
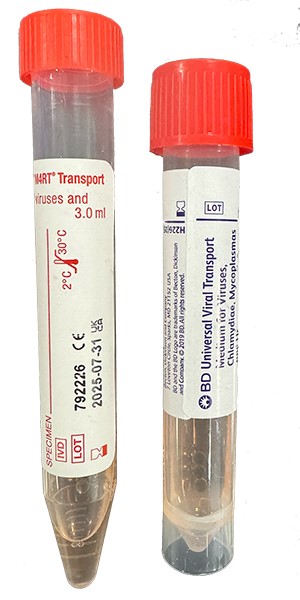
| Collect | Cervical, throat, urethral, rectal, eye swab or peritoneal fluid. Immediately place swab, fluid or washing in 3 mL universal transport medium such as M4, M4RT, M5, M6, UniTranz-RT, or UTM. Also acceptable for newborns: nasopharyngeal aspirate, swab or washing. Source of specimen is preferred. |
| Transport | Specimen in Chlamydia transport media (UTM) frozen. Source of specimen is preferred. |
| Stability | Ambient: 1 hour; Refrigerated: 48 hours; Frozen at -20°C: Unacceptable; Frozen (-70°C): 1 month |
| Unacceptable Conditions | Urine. Specimens in any media other than indicated. Dry swabs, wood swabs and calcium alginate swabs. |
| Schedule | Daily |
| Billing Code | 5011072 |
| CPTCode | 87110 Culture, 87140 Stain |
| Notes | Nucleic Acid amplification testing is recommended for detection of Chlamydia trachomatis from endocervical or urethral specimens. Refer to Chlamydia trachomatis by Amplified Detection (APTIMA). Specimen must be collected and transported with test-specific kit. Culture is recommended for Chlamydia trachomatis detection in suspected sexual abuse and for suspected failure of therapy. |
| Preferred Specimen Collection Device(s) | |
| Reference Interval | |
|
Culture negative for Chlamydia trachomatis. |
|